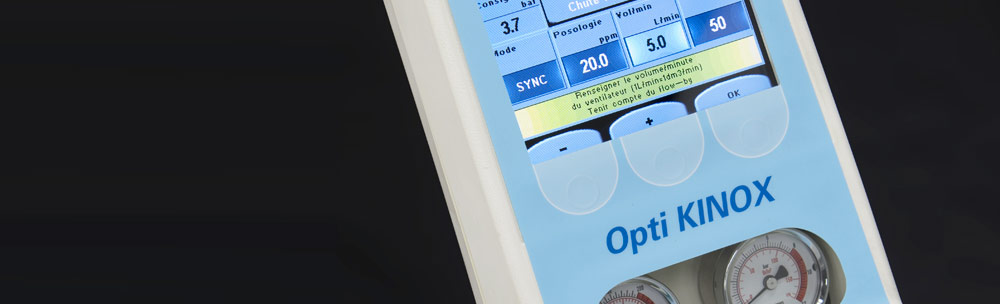
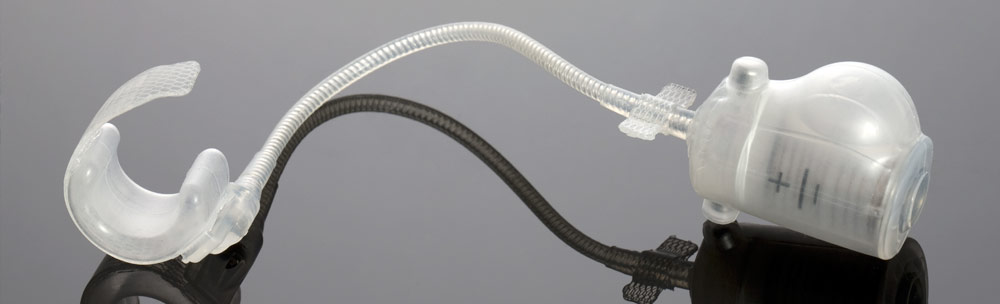
About Us
A supplier of micro-technical services in biomaterials, STATICE designs and produces innovative made-to-measure instruments, and supports their clients from the beginning of the project through to the final production line. STATICE is a manufacturing reference in the field of implants, medical instruments, and laboratory equipment. The medical instruments and other designs developed by STATICE are used in all industries where precision is of the utmost importance:
- Cardio-vascular
- Neurology
- Urology
- Orthopaedics
- Oncology
- Ophthalmology
- Instruments with minimal invasiveness etc...
STATICE was founded in 1978 by a group of engineers from the watch-making industry; at the time, they specialised in the production of made-to-measure micro-technology. The resulting outcome was two fields of specialisation: biomaterials and applied mechatronics for the medical industry, of which medical instruments and in vitro diagnostics. The R&D department then decided to double-up with the production site following suggestions and needs for their clients.
The R&D department has over 30 staff, of which Doctors and Engineers, a mechanical and electronics laboratory and testing area. Statice has an output of over 100 industrial projects per year, and together with their ¹SRC certification (¹Société Privée de Recherche sous Contrat - ¹A Private Company for Research Projects under Contract) granted by the BPIFRANCE/OSEO organism, and STATICE can undertake research projects over a medium and long-term range. STATICE is a regular partner in French and European industrial research projects which enables them to always be at the forefront of the most up-to-date technologies and innovations in their field of expertise; STATICE is recognised in Europe for their innovation skills.

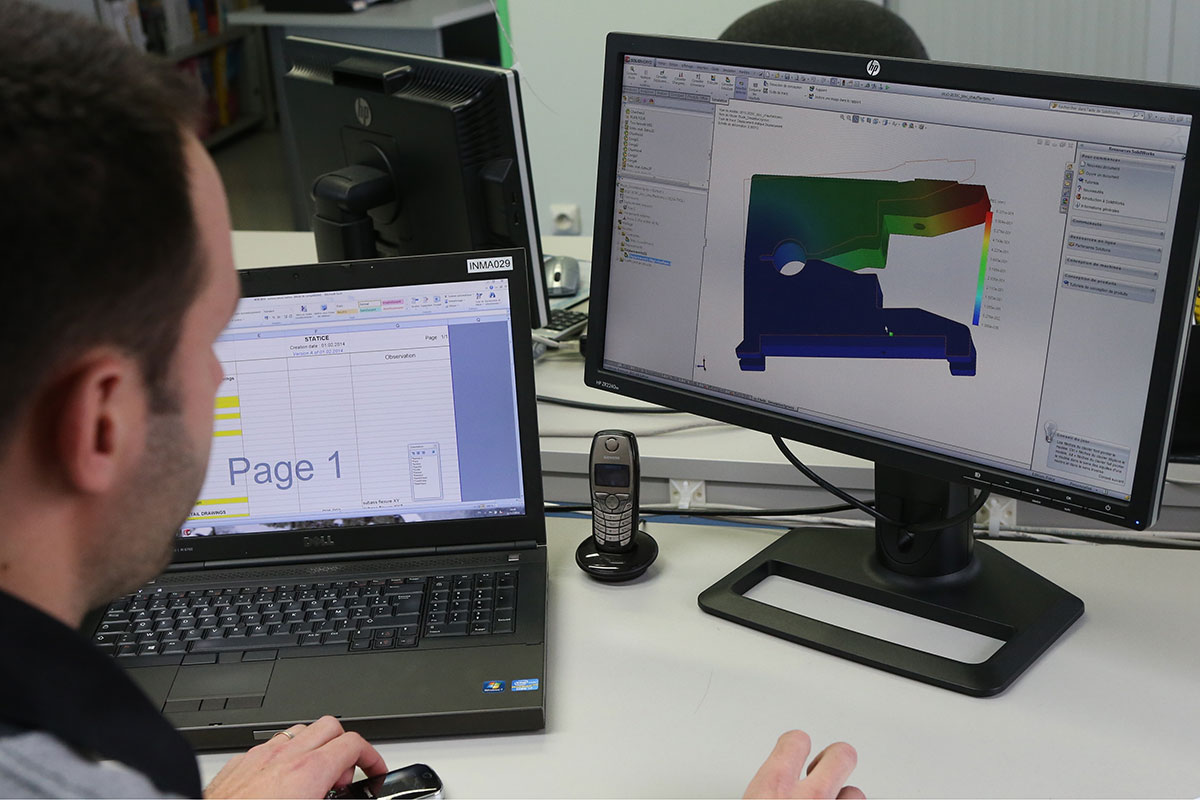
The production team has 70 staff who work in a controlled and secure environment with ISO5 and ISO7 certifications.
The manufacturing projects are ensured by a team of 4 who design and produce the required tools, check and control the ability to manufacture and produce a product via pre-testing under IQ/OQ/PQ, VMP, Gage R&R, and they prepare and edit the ²DMR (²Production Reference File). The production line is guided by an ERP Enterprise Resource Planning that ensures traceability with a barcode.
To date, both activities are inseparable, and enrich each other on a technical level.